ddPCR
Genomic and Molecular ServicesDroplet Digital PCR Applications for Cell and Gene Therapy Unrivaled Precision and Accuracy for Quality Manufacturing
Droplet Digital PCR (ddPCR) provides highly precise, absolute quantification of nucleic acid sequences by counting nucleic acid molecules encapsulated in discrete, volumetrically defined water-in-oil droplet partitions. This digital measurement is suitable for the analysis of both, in-process samples and final drug products, due to the inhibitor tolerant nature of Droplet Digital PCR. This insensitivity to formulation matrices enables the implementation of Droplet Digital PCR throughout the entire manufacturing process. Bio-Rad’s ddPCR systems utilize a simple workflow with minimal hands-on time while reducing sample replicates and user-to-user variability.
– Installation qualification/operational qualification (IQ/OQ) service
– Premier service offerings with rapid on-site support
Droplet Digital PCR Tools for Adeno-Associated Virus and Lentivirus Production in Gene Therapy
Developing effective and reproducible gene therapies requires the use of sensitive and robust testing methods, such as Droplet Digital PCR, to validate the quality of the therapeutic product. Bio-Rad’s portfolio of ddPCR Viral Quantification solutions offers an accurate, precise, and robust method to measure the viral titer in a sample. Customers may also use some of these solutions to perform quality checks on incoming plasmids and conduct biodistribution studies.
Plasmid Quality
- Vector Backbone Assays
- Gene of Interest Assays
Viral Titer and Vector Copy Number
- Vector Backbone Assays
- Gene of Interest Assays
Contaminant Testing
- Mycoplasma Detection
- Residual DNA Supermix
- Host Cell DNA (293) Detection (coming soon)
Biodistribution and Patient Monitoring
- Vector Backbone Assays
- Gene of Interest Assays
ddPCR Assays for AAV Viral Titer Quantification
MOgene Droplet Digital PCR (ddPCR) Assays for AAV viral titer utilize the precision and sensitivity of ddPCR technology to deliver an absolute count of AAV vector genome copies in your sample. These assays can be ordered through the Cell and Gene Therapy Design Engine and come in two varieties:
– Custom ddPCR Cell and Gene Therapy Assays generated for target gene of interest
– Ready-to-order ddPCR Expert Design Assays for common viral vector and
plasmid elements
Droplet Digital PCR Tools for CAR-T Development and Monitoring in Cell Therapy
Development, validation, and implementation of robust and accurate methods are vital to test the safety and efficacy of gene-modified cell therapies, such as Chimeric Antigen Receptor T (CAR-T) cells. Droplet Digital PCR offers absolute quantification of transgene copy number in a sample. This method can be used to effectively quantify CAR copy numbers in transfected or transduced T cells or count the number of cells that contain the transgene of interest. This is especially important for transgenes that cannot be detected by antibodies. The ddPCR Whole Cell DNA Workflow is a reproducible and easy way to measure the percentage of edited cells with minimal input cells, which can be applied at multiple steps during the cell therapy manufacturing process.

Vector Copy Number
- Axi-cel Assay
- Axi-cel and Tisa-cel Universal
Percent Modified Cells
- Whole Cell DNA Workflow
- Axi-cel Assay
- Axi-cel and Tisa-cel Universal

Contaminant Testing
- Mycoplasma Detection
- Residual DNA Supermix
- Host Cell DNA (293) Detection (coming soon)

Biodistribution and Patient Monitoring
- Axi-cel Assay
- Axi-cel and Tisa-cel Universal
The ddPCR Whole Cell DNA Workflow:
- Accurately measure the percentage of modified cells at the DNA level
- Does not require surface expression or cell staining
- Robust and sensitive detection enables low input cell requirement (100–1,000 cells)
- Easy, reproducible workflow and analysis makes it a scalable solution
Experience the Advantages of Droplet Digital PCR
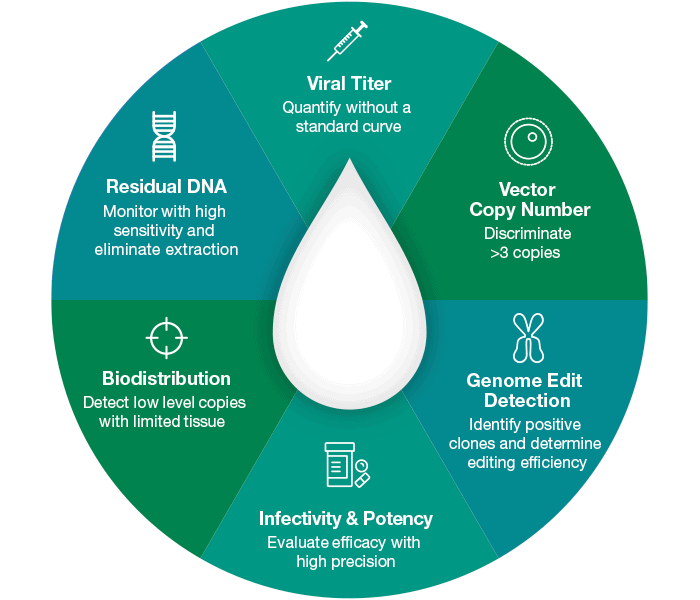
Unrivaled Precision and Accuracy for Quality Manufacturing
droplet digital PCR
MOgene uses QX200 AutoDG ddPCR system (Bio-Rad Labs Inc.) which generates water-oil emulsion droplets that partitions the single PCR reaction into 20,000 droplets and the presence or absence of the fluorescent PCR product in each of these droplets is counted to evaluate the target concentration. This ability to evaluate the DNA abundance in tens of thousands of individual reactions increases the precision as well as the sensitivity of these assays. MOgene offers both custom and commercially available assays for a wide variety of applications such as gene expression and genetic variation analysis. We manage all aspects of the project from assay design and nucleic acid isolation, to data analysis.
QX200 AutoDG ddPCR system
Using Bio-Rad’s QX200 AutoDG ddPCR system allows MOgene to customize the experiments to suit your experimental requirements.
SERVICE FEATURES AND OPTIONS
- PCR primers and probe design
- Nucleic acid isolation from a wide variety of samples
-
Assay optimization with proper controls included
- Data analysis
- Report with project description
Quality and safety
The Quality Commitment From MOgene
Regulatory Services
MOgene has a state-of-the-art, CLIA-certified facility with an established reputation of complying with relevant national and international GxP compliance regulations.
- Audit ready for clients with routine internal quality audits
- GLP compliance per FDA 21 CFR Part 58 and Part 11
- Active CAPA program for process improvement
- CLIA Certification
- USDA Certification
Quality Management System
MOgene has a robust Quality Management System (QMS) detailed in a manual that ensures compliance for quality monitoring and improvement.
- Rigorous sample management process
- Transparent and routine communications on project timelines and deliverables
- Complete documentation of laboratory procedures (SOPs), 100% QC of data
BioSafety
Our Biosafety practices enable us to provide genomics services for early R&D to market release studies for pathogens that require BSL-2+ facilities.
- Higher levels of lab safety for antimicrobial, human and animal health vaccine development
- Dedicated BSL-2+ Lab and pre-amplification room for your clinical studies
- Trained personnel to handle BSL-2+ level agents
- UNIDIRECTIONAL workflow
Security and Data Protection
MOgene considers safety and confidentiality a top priority for our client’s data.
- 24/7/365 temperature and humidity monitoring
- Individually coded key access to labs
- Secured access to data records
- Daily data back-up
OUR HISTORY SPEAKS FOR ITSELF
Supporting Genomic Studies Since 2004
Industry Leading Experienced Team
More Than 150 Citations in Peer-reviewed Journals