PACBIO sequencing
Genomic and Molecular Services
PACBIO SEQUENCING
MOgene now offers all sequencing services on the PacBio Sequel sequencing system. The Sequel system, a next generation of SMRT (Single-molecule, real-time) sequencing system, offers much higher throughput and more scalability than the Second-Generation Sequencing (SGS) technologies, making it well-suited for unsolved problems in genome, transcriptome, and epigenetics research.
The highly-contiguous de novo assemblies using PacBio sequencing can close gaps in current reference assemblies and characterize structural variation (SV) in personal genomes. With longer reads, we can sequence through extended repetitive regions and detect mutations, many of which are associated with diseases. Moreover, PacBio transcriptome sequencing is advantageous for the identification of gene isoforms and facilitates reliable discoveries of novel genes and novel isoforms of annotated genes, due to its ability to sequence full-length transcripts or fragments with significant lengths.
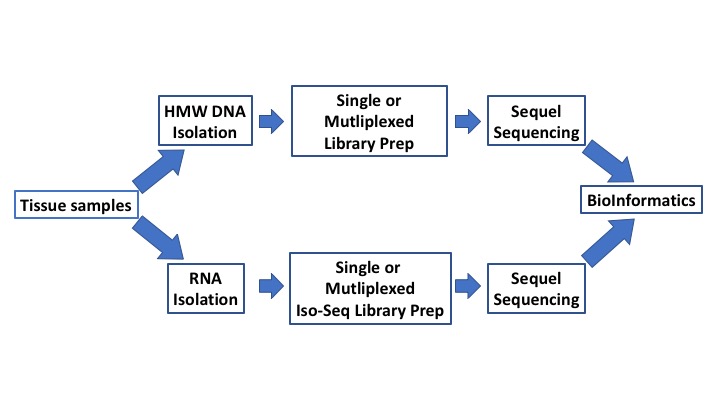
Additionally, PacBio’s sequencing technique provides information that is useful for the direct detection of base modifications, such as methylation. In addition to using PacBio sequencing alone, many hybrid sequencing strategies, such as combination of PacBio sequencing and Illumina data, have been developed to make use of more accurate short reads in conjunction with PacBio long reads. In general, hybrid sequencing strategies are more affordable and scalable especially for small-size laboratories than using PacBio Sequencing alone. The advent of PacBio sequencing has made available much information that could not be obtained via SGS alone.
Applications include
- de novo Assembly
- Exomes and whole genomes
- Targeted Sequencing
- Base modifications
- Isoform sequencing detection
- Amplicon\COSMID sequencing
Areas include
- Human
- Plant & Animal
- Microbiology
PacBio Sample Submission Guidelines
Please review following documents for DNA or RNA (IsoSeq) sample submission and shipping guidelines.
Quality and safety
The Quality Commitment From MOgene
Regulatory Services
MOgene has an established reputation of complying with relevant national and international quality compliance regulations.
- Audit ready for clients with routine internal quality audits
- GLP compliance per FDA 21 CFR Part 58 and Part 11
- Active CAPA program for process improvement
- CLIA Certification
- USDA Certification
Quality Management System
MOgene has a robust Quality Management System (QMS) detailed in a manual that ensures compliance for quality monitoring and improvement.
- Transparent and routine communications on project timelines and deliverables
- Complete documentation of standard operating procedures (SOPs) and 100% QC of data
- Rigorous sample management process
BioSafety
Our Biosafety practices enable us to provide services for early R&D and market release studies for pathogens that require BSL-2+ facilities.
- Higher levels of lab safety for antimicrobial, human and animal health vaccine development
- Dedicated BSL-2+ Lab and pre-amplification room for your clinical studies
- Trained personnel to handle BSL-2+ level agents
- UNIDIRECTIONAL workflow
Security and Data Protection
MOgene considers safety and confidentiality a top priority for our client’s data.
- 24/7/365 temperature and humidity monitoring
- Individually coded key access to labs
- Secured access to data records
- Daily back-up of data
OUR HISTORY SPEAKS FOR ITSELF
Supporting Genomic Studies Since 2004
Industry Leading Experienced Team
More Than 150 Citations in Peer-reviewed Journals